|
published in: "The Journal
of the Orders and Medals Society of America", volume 51,
number 4, pages 30-32, and received the
OMSA Literary Medal 2001. The collecting and study of orders, decorations, and medals is an interdisciplinary enterprise that requires some knowledge of history, art, and numismatics. The knowledge about fakes and how they are technically made is especially important. Some fakes are easy to spot, but others are so deceptive that only an expert on the award in question can see through the fraud. Among the most deceptive fakes are those produced by the electroplating process; however, these fakes can often be detected by collectors who have no particular award expertise but do know how they were made.
Illustrated are the first
and second issues of the Nassau Life-Saving Medal. The
first issue was awarded on 13 February 1843 by Nassau’s
Duke Adolph. This silver medal, which is 30mm in
diameter, was designed by Christian Zollmann. Is It was
not meant to be worn. On the obverse is a large profile
head of a young Duke Adolph facing the right, and
encircling the profile is the inscription ADOLPH
HERZOG ZU NASSAU in raised letters. The reverse is
plain except for the inscription FUR/RETTUNG/AUS/LEBENSGEFHR
(literally, “For Rescue Out of Life-Threatening
Danger”) in the bottom. A total of 54 first-issue medals
were struck and awarded.
In 1865, 30 silver life-saving medals of a new design and also 30mm in diameter were struck. The 1843 reverse was retained, but the obverse now featured the profile of an older looking Duke Adolph facing to the left, and the name of the die sinker, “KORN,” was added at the base of the profile in small raised letters. Moreover, the new design incorporated a suspension device and solid red ribbon so that the medal could be worn. Only 5 of the second issue were awarded, and the remaining 25 medals were melted down in late 1866.
Thus both issues, and in
particular the second issue, are extremely rare and
highly prized by collectors of the awards of the German
states. It is a small wonder, then that someone took the effort
to make the illustrated second issue, which is a fake
produced by electroplating or -forming.
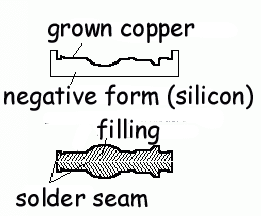
Before closely examining this fake, it is first necessary to briefly describe the electroplating process. The principle was discovered by Faraday in 1833, and the process was patented in England in 1840. The purpose of electroplating is to transfer and bind the atoms of one metal to the surface of another metal or to the surface of an object coated with a conductive material. Electroplating requires a DC power supply, often a battery, a chemical bath called the “electrolyte,” and an “anode” or positive pole at one end of the bath and a “cathode” or negative pole at the opposite end. When the anode is connected to the positive pole of the power supply and the cathode to the negative pole of the supply, electrons stream from the metal of the anode to the cathode. This stream is part of the electrical current that flows from the positive pole of the power supply to the anode, through the electrolyte to the cathode, and then from the cathode to the negative pole of the power supply, thus completing the circuit. Electricity is merely a stream of electrons through a conductor, which can be either a metal wire or the chemicals in a plating bath.
The shedding of electrons
from the anode simultaneously produces positively
charged atoms or “ions” of the metal of the anode. The
ions are attracted or pulled to an excess of electrons
gathering at the cathode. These ions bind with free
electrons at the cathode producing neutral atoms of the
metal that attach to the cathode to form the plate. It
is important to note that the source of the plating
metal need not be the anode. Electroplating is often
accomplished by using a nonreactive anode, such as
platinum, and an electrolyte containing a salt of the
plating metal. The chemistry is the same, except that the
source of the plating metal is the electrolyte instead
of the anode. Of course, the chemical reaction in the
electroplating process is more complicated than suggested by my simple explanation, and several important technical aspects must be considered, such as
the duration of the process and the composition of the
electrolyte, to achieve the desired consistency and
thickness of the plate.
Electroplated fakes are typically very difficult but not impossible to detect. The detail can be perfect, and the surfaces are as smooth as the original, unlike cast fakes, which typically have porous surfaces. In addition, a filler can be used inside the shell that gives the fake the same weight as the original. Nonetheless, the process itself provides knowledgeable collectors with certain indicators that can be used to determine whether or not a particular medal has been produced by electroplating. Consistent application of the following four indicators should greatly aid collectors in avoiding the misery brought by the purchase of one of these clever fakes:
First and foremost, it
must be remembered that the fake was not made as one
piece but consists instead of two halves that were
soldered together. This means that despite the best
efforts of the counterfeiter to polish out he solder
seam, a portion of the tell-tale seam almost always
appears on the edge of the medal. That portion of the
seam visible on the edge of the fake second-issue Nassau
Life-Saving Medal is shown in the enlargement below.
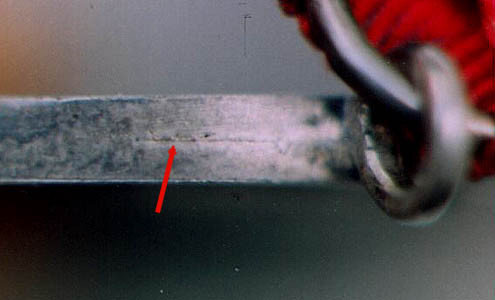 Secondly, the fake “sounds” different. Lead is often used to fill the inside of the fake, and sometimes, antimony is added to the lead to give the fake the same weight as the original. However, when tapped by a hard object, the fake with its lead filler sounds “dead” compared to a solid silver piece, which has a clear metallic sound. Thirdly, collectors should carefully examine the medal for areas where the silver plate has worn off and the copper plate underneath shows through. Simply put, medals struck in solid silver can’t have signs of copper. Finally, one must also carefully look at the medal’s detail. A fake produced by electroplating can have flawless detail, but this in not always the case because the surface of the fake is only as good as the surface of the mold. Very often, there are tiny deficiencies in the mold that appear in the final product. In the next enlargement, several problems are evident in the letters of the die sinker’s name on the obverse of the fake second-issue Nassau Life-Saving Medal.

Fakes produced by
electroplating are much harder to detect than cast
fakes, and counterfeiters have been greatly aided by a
lack of knowledge by both dealers and collectors of the
electroplating process and its indicators. To make
matters worse, electroplated fakes are appearing in the
market in increasing numbers. I remember from my days as
a young collector in Germany that original German World War I
aviation badges were almost impossible to find. There may be one original pilots badge but 100 electroformed forgeries.
© A. Schulze Ising,
VII/00
|